CONCLUSION
Philadelphia chromosome negative acute lymphoblastic leukemia (Ph- ALL) boasts contradicting survival rates given its binodal age distribution. While children and adolescents post 5-year overall survival (OS) rates of 89%, the stark contrast of 5-year OS of 20.4% in older adults greater than 65 years, makes this a challenging population to treat. Currently, there is no standard of care for the treatment of ALL with various regimens utilized dependent on age, co-morbidities, performance status, as well as physician/institute preference. One regimen that is commonly used is a combination of targeted therapies along with reduced intensity chemotherapy (inotuzumab ozogamicin, rituximab (if CD20+), blinatumomab and mini hyper fractionated cyclophosphamide, vincristine, and dexamethasone [Mini-HCVD]), which has demonstrated both improvements in survival while reducing treatment related toxicity. Another commonly accepted approach is a modified Berlin-Frankfurt-Munster (BFM) backbone (i.e., E1910). Despite the available options, there is a lack of comparison of these regimens. To answer this unanswered question, we compared the characteristics, outcomes, and toxicities of older adults with ALL treated with either Mini-HCVD or E1910 at our academic medical center.
Medical records were retrospectively reviewed at Roswell Park Comprehensive Cancer Center to identify patients diagnosed with Ph- ALL treated with Mini-HCVD or E1910 between January 1 2016 - May 31 2022. Those with relapsed/refractory ALL or received E1910 in the setting of a clinical trial were excluded from the study. Demographics, disease-specific variables, and overall outcomes were collected in accordance with the institutional review board approved protocol. Primary outcome was 3-year overall survival rate, while secondary outcomes included best response in first 3 months, minimal residual disease (MRD) status at 3 months, 30-day mortality, as well as the frequency and incidence of toxicities per cycle. Best response was defined as less than 5% blasts on bone marrow biopsy with complete (CR) or incomplete (CRi) absolute neutrophil or platelet count recovery. MRD status was assessed via flow cytometry and next generation sequencing using ClonoSEQ®. Adverse events and their severity were characterized according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Differences in OS between two groups were examined by log-rank test. Propensity scores were obtained based on the baseline variables that with potentially imbalanced distributions (p<0.1). MRD status and response to treatment were compared using Fisher's exact test. Toxicities were summarized as frequencies for each treatment cycle. The incidence of grade 3-4 toxicities was defined as the number occurrence per treatment cycle and were compared using Poisson regression.
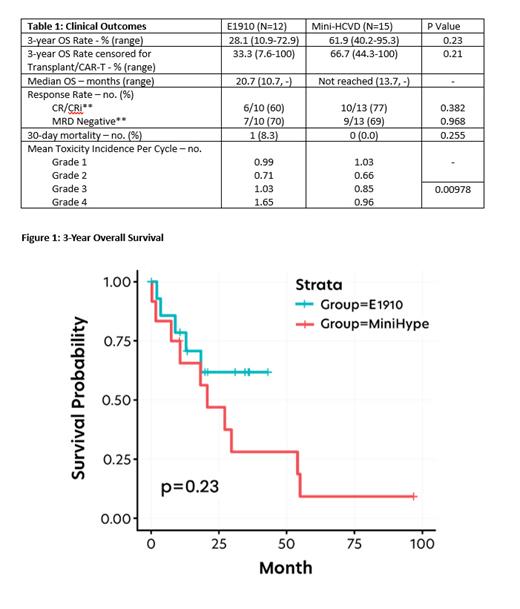
27 patients were included, with 15 (55.5%) receiving Mini-HCVD and 12 (44.5%) receiving E1910. Median age was 70 years (49-83), in Mini-HCVD arm and 63 years (47-74), in E1910 arm. Three-year OS was 61.9% for those who received Mini-HCVD (95% CI 40.2-95.3) and 28.1% for those who received E1910 (95% CI 10.9-72.9; p=0.23). In Mini-HCVD 77% and 69% of patients achieved CR/CRi and MRD negative status in the first three months, compared to 60% and 70% in E1910. Only 13% of patients in Mini-HCVD proceeded to transplant or CAR-T, compared to 58% in E1910 (p=0.0198). The most common treatment-related adverse events in both arms were cytopenias. Febrile neutropenia occurred in 47% of Mini-HCVD patients compared to 83% in E1910. There was no difference in rates of infections between arms. Mean incidence of grade 3/4 toxicity per cycle was 0.85/0.96 with Mini-HCVD and 1.03/1.65 with E1910 (p=0.0098).
This retrospective single institute study demonstrates that the clinical outcomes of Mini-HCVD plus immunotherapeutic agents in older adults with Ph- ALL are similar to modified BFM backbone with reduced incidence of toxicity. While limited by small numbers, these results suggest that reduced intensity chemotherapy in combination with novel targeted agents could represent a new standard of care regimen in this historically difficult to treat patient population.
Disclosures
Przespolewski:Jazz Pharmaceuticals: Research Funding. Thompson:Novartis: Research Funding; Bristol Myers Squibb: Research Funding. Griffiths:Apellis Pharmaceuticals: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Abbvie: Consultancy; Alexion Pharmaceuticals: Consultancy, Research Funding; S. Karger Publishing: Honoraria; Artis Ventures: Membership on an entity's Board of Directors or advisory committees; AstraZeneca Rare Disease: Consultancy, Research Funding; Picnic Health: Membership on an entity's Board of Directors or advisory committees; Takeda Oncology: Consultancy; Blueprint Medicines, Inc: Research Funding; Taiho Oncology: Consultancy; Celldex Therapeutics: Research Funding; American Society of Hematology: Honoraria; Novartis: Consultancy, Research Funding; Partner Therapeutics: Consultancy; Physicians Educational Resource: Honoraria; NextCure, Inc: Research Funding; Genentech, Inc.: Consultancy, Research Funding; AAMDSIF: Honoraria; CTI Biopharma: Consultancy; Astex Pharmaceuticals: Research Funding; MediCom Worldwide, Inc.: Honoraria; MDS International Foundation: Honoraria; Medscape: Honoraria; Vera and Joseph Dresner Foundation: Membership on an entity's Board of Directors or advisory committees. Wang:Kura Oncology: Speakers Bureau; Dava oncology: Speakers Bureau; Takeda: Consultancy; PharmaEssentia: Consultancy; Pfizer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Kite: Consultancy, Speakers Bureau; Jazz: Consultancy; GlaxoSmithKline: Consultancy; Gilead: Consultancy; BMS: Consultancy; Astellas: Consultancy, Speakers Bureau; Abbvie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal